Products
Product information
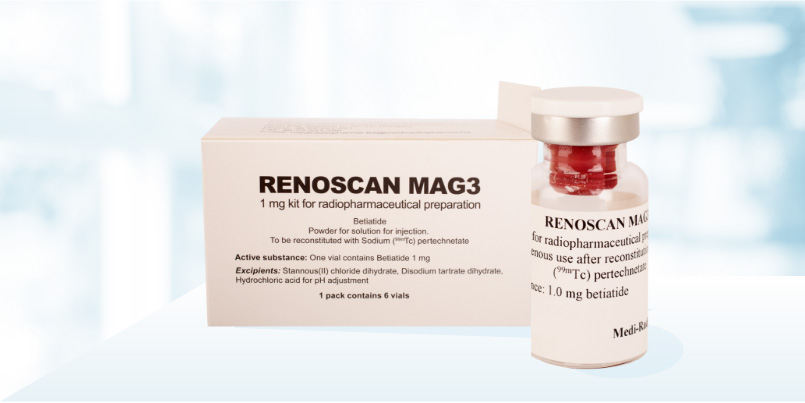
Renoscint MAG3 1 mg

Active substance
Betiatide To be used with sodium (99mTc) pertechnetate for the preparation of the diagnostic agent: Technetium (99mTc) tiatide.
Indications
After reconstitution and labelling with sodium (99mTc) pertechnetate solution, the diagnostic agent technetium (99mTc) tiatide may be used intravenously for the evaluation of nephrological and urological disorders in particular for the study of morphology, perfusion, and function of the kidney and characterisation of urinary outflow.
Excipients
- Disodium tartrate dihydrate
- Stannous (II) chloride dihydrate
- Hydrochloric acid for pH adjustment
Dose for adults
37-185 MBq, depending on the pathology to be studied and the method to be used. Studies of renal blood flow or transport through the ureters generally require a larger dose than studies of intra-renal transport, whereas renography requires smaller activities than sequential scintigraphy.
Labelling volume
10 ml
Labelling activity
maximum 2960 MBq
Storage of cold kit
18 months from date of manufacturing,
Store in a refrigerator (2°C – 8°C)
Storage of labelled compound
8 hrs,
Do not store above 25°C
Package size
1 pack contains 6 vials
Sample package: 2 vials
Registration numbers
Hungary: OGYI-T-23275/01 (1x)
OGYI-T-23275/02 (6x)
Czech Republic: 88/832/16-C
Denmark: DK R 58417
Italy: AIC 045669013
United Kingdom: PL 27151/0001
Austria: 438272
Germany: 98671.00.00
Spain: 82909
Poland: 24615
Marketing Authorization Holder
Medi-Radiopharma Co., Ltd.
2030 Érd, Szamos u. 1012., Hungary
Sales Information
For more information: